Oracle Quarterly
Patches and FDA Validation

GoSaaS has provided software application validations to several manufacturing Companies across industry verticals.
What is quarterly patches and fda validation?
Many companies, however, struggle to keep up with quarterly testing cycles and are missing the opportunity to achieve new, high-impact business outcomes through their Oracle investments.
To truly harness the value of Oracle, companies need a provider that can transcend traditional support services and guide them in their ongoing strategic journey. GoSaaS’ proven tools and methods operationalize & automate quarterly release planning and execution activities while preparing you for what’s ahead – Allowing you to focus on enablement of new digital capabilities and innovation.
Are you facing these challenges?
Difficulty with resource/ramping productivity during release cycles
Uncertainty and challenges arise from the extensive release documentation, leaving questions about its applicability
Irregular adoption of new features that maybe holding you back
The effort put into supporting unnecessary extensions or applications
Absence of a strategic plan that takes into account upcoming releases.
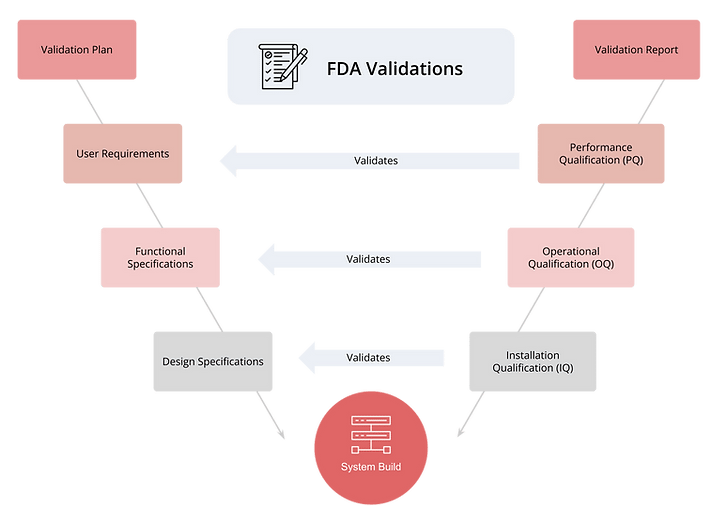
Validations Include:
Installation Qualification (IQ), Operational Qualification (OQ) and Performance Qualification (PQ)
Validation scripts that have been developed and delivered based on FDA andvindustry standards
Scripts are provided for each module of Oracle Product Lifecycle Management (PLM) Cloud, GoSaaS Environmental Governance & Compliance (EG&C) and GoSaaS Employee Training System (ETS)
GoSaaS has provided resources/people for running the scripts and support when customers prefer to run the scripts themselves
GoSaaS has options for automatically performing ongoing validations or providing updated scripts in the case of software application updates and upgrades. For example: Oracle Product Lifecycle Management (PLM) Cloud receives quarterly updates for which customers may or may not opt in. GoSaaS performs the validations as required by the FDA and other regulatory bodies and reports the results automatically.
Value add services
Tailored release notes
Functionality gap assessment
Customization rationalization
Innovation workshopping
Flexible commercial terms for support contracts
Validation Pack Creation Workflow
GoSaaS Validation scripts are developed and delivered based on FDA and industry standards. We provide staff augmentation for running the scripts and support when customers prefer to run them themselves.
Our process workflow is as follows:
-
PLM/Product Design finalized
-
Validation Plan Created
-
SRS Creation
-
Assess risks
-
Create URS/FRS/TM
-
Provide OQ
-
Create test cases for IQ
-
Create test cases for PQ
-
Provide/Execute Documents
Can currently offer automated testing for the PLM Performance Qualification and ETS Operational Qualification.